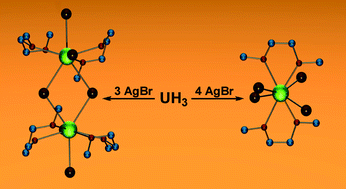
Addition of 2 equiv of I2 to a stirring suspension of UH3 in Et2O results in vigorous gas evolution and the formation of UI4(OEt2)2 (1), which can be isolated in good yields as an air- and moisture-sensitive brick-red powder. Addition of 3 equiv of AgBr to UH3 in DME produces UBr3(DME)2 (2), while addition of 4 equiv of AgX to UH3 in DME–CH2Cl2 provides UX4(DME)2 (X = Br, 3; Cl, 4). Similarly, the reaction of 4 equiv of AgOTf with UH3 in neat DME generates U(OTf)4(DME)2 (5). Each of these reactions proceeds with the evolution of hydrogen. Complex 1 can also be generated by reaction of 4 equiv of Me3SiI with UCl4 in Et2O. All complexes were fully characterized, including analysis by X-ray crystallography.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...