A family of sensitive energetic salts of the 5-nitrotetrazolate anion with alkali metal cations (Li+1, Na+2, K+3, Rb+4 and Cs+5) were synthesized either by the digestion of an acid copper salt of 5-nitrotetrazole with a suitable metal hydroxide, or alternatively by reaction of ammonium 5-nitrotetrazolate with a suitable metal base (MOH, MHCO3 or M2CO3) in aqueous or alcoholic solution. All the compounds were characterized by analytical methods (elemental analysis and mass spectrometry) and spectroscopic methods (NMR and vibrational spectroscopy). The lighter metal salts 1 and 2, incorporate three and two crystal water molecules in the structure, respectively, whereas the heavier alkali metal derivatives form anhydrous species, and thus showed enhanced sensitivity to friction and shock. In addition, the crystal structure of each of the new materials was determined by X-ray diffraction techniques (1 and 3: monoclinic, P21/c; 2: triclinic, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ; 4: monoclinic, Cc and 5: monoclinic, C2/c). The thermal stability of compounds 1–5 was assessed by differential scanning calorimetry (DSC) measurements showing significant thermal stability. Lastly, the energies of combustion of 1 and 2 were measured experimentally using oxygen bomb calorimetry (1, −1340(15) cal g−1 and 2, −1200(20) cal g−1) and was used to calculate their standard molar heats of formation (1, −610(55) kJ mol−1 and 2, −360(65) kJ mol−1).
; 4: monoclinic, Cc and 5: monoclinic, C2/c). The thermal stability of compounds 1–5 was assessed by differential scanning calorimetry (DSC) measurements showing significant thermal stability. Lastly, the energies of combustion of 1 and 2 were measured experimentally using oxygen bomb calorimetry (1, −1340(15) cal g−1 and 2, −1200(20) cal g−1) and was used to calculate their standard molar heats of formation (1, −610(55) kJ mol−1 and 2, −360(65) kJ mol−1).
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ; 4: monoclinic, Cc and 5: monoclinic, C2/c). The thermal stability of compounds 1–5 was assessed by
; 4: monoclinic, Cc and 5: monoclinic, C2/c). The thermal stability of compounds 1–5 was assessed by 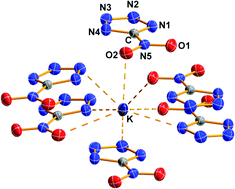

 Please wait while we load your content...
Please wait while we load your content...