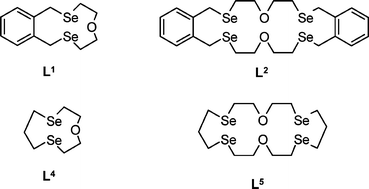
Treatment of O(CH2CH2SeCN)2 with Na in NH3(l), followed by dropwise addition of a thf solution of o-C6H4(CH2Br)2 at −40 °C leads to formation of three mixed Se/O-donor macrocycles which are separable by column chromatography, the [1 + 1] species L1, the [2 + 2] ring L2 and the [3 + 3] ring L3, of which L2 is by far the major species. Using the same starting materials, but in a high dilution cyclisation at room temperature with NaBH4 in thf/EtOH gives exclusively the [1 + 1] ring, L1. The saturated ring Se/O-donor macrocycles, L4 and L5 are obtained by simultaneous dropwise addition of solutions of O(CH2CH2SeCN)2 and Br(CH2)3Br to NaBH4 suspended in thf/EtOH. The small tridentate Se2O-donor ring, L4, is again the dominant product under these conditions (71%), although the more flexible precursors in this reaction also give rise to the larger Se4O2-donor ring, L5, as a by-product in 8% yield. These compounds are readily separated and purified by column chromatography (ethyl acetate:hexane, 1:19). The new macrocycles have been characterised by 1H, 13C{1H} and 77Se{1H} NMR spectroscopy and mass spectrometry, together with crystal structures of L1 and L2. Complexes of L1 and L2 with late transition metals (Pd(II), Pt(II), Cu(I) and Ag(I)) are also described.

 Please wait while we load your content...
Please wait while we load your content...