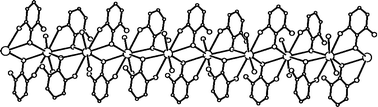
Starting material KN(H)C6H3-2,6-F2 was prepared via a transamination reaction from KNH2 and 2,6-F2C6H3NH2 in THF and crystallized from 1,4-dioxane (diox) as the three-dimensional polymer [(diox)1.5K{N(H)-2,6-F2C6H3}·diox0.5]∞ (1). The metathesis reaction of (THF)4CaI2 with KN(Me)Ph in THF yields monomeric (THF)4Ca[N(Me)Ph]2 (2) with a nearly linear N–Ca–N moiety of 179.84(8)°. The metathesis reaction of (THF)4CaI2 with KN(H)Mes yields trinuclear (THF)6Ca3[N(H)Mes]6 (3) with a linear Ca3 fragment and bridging 2,4,6-trimethylphenylamido groups. The reaction of 1 with (THF)4CaI2 gives dinuclear (THF)5Ca2[N(H)-2,6-F2C6H3]4·2THF (4) with three bridging and one terminally bound 2,6-difluorophenylamide. A similar reaction of (THF)5SrI2 with KN(H)-2,6-F2C6H3 yields dinuclear (THF)6Sr2[N(H)-2,6-F2C6H3]3I·THF (5) in which the iodide anion binds terminally. This iodide ligand cannot be substituted as easily by excess KN(H)-2,6-F2C6H3. The metathesis reaction of (THF)5BaI2 with KN(H)-2,6-F2C6H3 leads to the formation of [(THF)2Ba{N(H)-2,6-F2C6H3}2]∞ (6) which crystallizes as a one-dimensional polymer with bridging 2,6-difluorophenylamide anions and additional Ba–F-bonds.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...