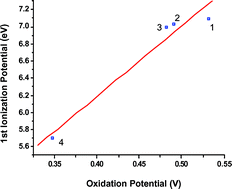
The electronic communication between two redox centres through a Schiff base complex has been investigated in a series of ethylenediimine-bis(1-ferrocenyl-1,3-butanedionate) complexes of Zn(II) 1, Cu(II) 2, Ni(II) 3 and Co(II) 4. Cyclic voltammetry experiments of 1 and 2 exhibit a unique two-electron reversible oxidation wave, whereas in the case of 3 and 4 two and three one-electron oxidation processes are, respectively, observed. These results suggest some electronic interaction between the iron atoms of the ferrocenyl groups. DFT calculations carried out on model complexes show that for all the studied compounds the removal of the first two electrons corresponds to the oxidation processes of the iron centres in the weakly coupled ferrocenyl termini. The electronic communication between the two iron centres increases on going from 1 to 4. Finally, a re-indexation of the bands observed in the UV-Visible spectra has been carried out using TDDFT calculations.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...