A truce on the Smiles rearrangement: revisiting an old reaction—the Truce–Smiles rearrangement
Abstract
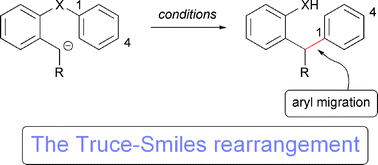
The Smiles rearrangement is the intramolecular nucleophilic aromatic substitution reaction incorporating a heteroatom as the nucleophilic component and an activated electrophilic

 Please wait while we load your content...
Please wait while we load your content...