Functionalization of diazines and benzo derivatives through deprotonated intermediates
Abstract
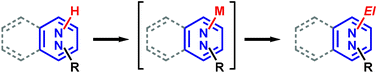
This critical review targets as a readership researchers generally oriented toward organic synthesis and in particular those active in heterocyclic chemistry. Diazines and benzo derivatives can undergo deprotonative metalation provided that the base is properly chosen. Metalation reactions of a large range of substrates can be performed using hindered lithium dialkylamides such as lithium 2,2,6,6-tetramethylpiperidide. Subsequent reactions with electrophiles open an entry to a great variety of building blocks, notably for the synthesis of biologically active compounds. (83 references)

 Please wait while we load your content...
Please wait while we load your content...