The chemistry of gold as an anion†
Abstract
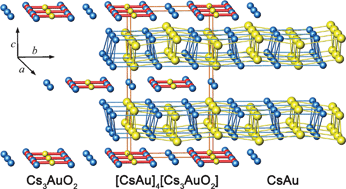
Due to relativistic and classical shell structure effects, the 6s orbital of gold is significantly contracted and energetically stabilized. This is reflected by a strikingly high electron affinity, and a distinct tendency to adopt negatively polarized valence states. This tutorial review focuses on the chemistry of gold as an anion, displaying the integral ionic charge number of 1−. Two synthetic approaches to compounds containing monoatomic gold anions have become available: (1) reacting
- This article is part of the themed collection: Gold - Chemistry, Materials and Catalysis

 Please wait while we load your content...
Please wait while we load your content...