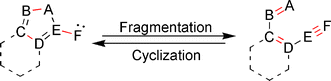
The fragmentation of a 5-membered heteroaromatic ring to afford a conjugated ene–ene–yne skeleton, and the corresponding reverse process, cyclization of the hetero-ene–ene–yne motif to generate a variety of heterocyclic systems, are the subject of this review. These synthetically useful reactions, which proceed through a coarctate/pseudocoarctate mechanistic pathway, are unique in that they involve the generation of either a carbene or nitrene intermediate, and provide access to hard to obtain heterocyclic or ene–ene–yne structures. While fragmentation of heteroaromatic rings containing a exocyclic carbene or nitrene has been well documented in the literature for over 40 years, the use of hetero-ene–ene–yne precursors to synthesize heterocycles is a relatively new approach that is generating much interest in the literature. This review highlights both the synthetic and mechanistic aspects of these unique reactions.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...