Combinatorial screening of PtTiMe ternary alloys for oxygen electroreduction
Abstract
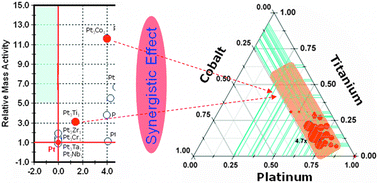
The catalytic oxygen electroreduction properties of PtTiMe (Me = Co, Cr, Cu, Fe, Mn, Mo, Ni, Pd, Ta, V, W and Zr) ternary alloys were studied using an in-house developed thin film based combinatorial high throughput method. Libraries containing discrete alloy compositions were fabricated by plasma co-sputtering and the resulting alloys were electrochemically screened by the hydrodynamic rotating disk electrode technique. Candidate
- This article is part of the themed collection: Electrocatalysis: theory and experiment at the interface

 Please wait while we load your content...
Please wait while we load your content...