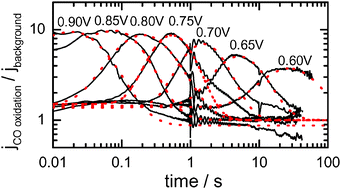
The assumption that “OHads” or other oxygen containing species is formed on polycrystalline or nanoparticulate platinum through a fast and reversible process at relatively low potentials is often made. In this paper we discuss the implications of this assumption and the difficulty in reconciling it with experimental phenomena. We show how presenting chrono-amperometric transients as log–log plots for potentials steps in the presence and absence of an adlayer of carbon monoxide on polycrystalline platinum is particularly useful in understanding the time evolution of the CO oxidation reaction. When using log–log plots a clear power law decay can be observed in the transients both in the presence and absence of an adlayer of carbon monoxide. We explain this as an extension of current theory, such that the rate determining step in both cases is the formation of a hydrogen bonded water-OHads network, strongly influenced by anions, and that COads oxidation occurs, at least in part by the diffusion of OHads through this network. We hypothesize that, at low potentials the formation of OHads at active sites is fast and reversible but that transport of OHads away from those sites may be rate limiting. The assumption that overall OHads formation on platinum is fast and reversible is therefore highly dependent upon the platinum surface and the experimental conditions and it may not be appropriate for polycrystalline surfaces in sulfuric acid. Therefore, although the formation of OHads on platinum in the absence of strongly adsorbing anions on ‘ideal’ surfaces is almost certainly fast and reversible, on realistic fuel cell relevant surfaces under non-ideal conditions this assumption cannot be made, and instead the formation of an OHads adlayer may be somewhat slow and is associated with the formation of hydrogen bonded water-OHads networks on the surface. We expect this to be a more realistic description for what occurs during COads oxidation on fuel cell relevant catalysts which are highly heterogeneous and which have a highly defective surface.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...