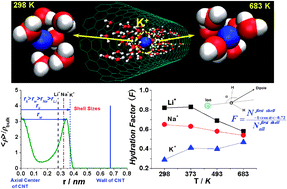
Molecular dynamics simulations have been performed to investigate the hydration of Li+, Na+, K+, F−, and Cl− inside the carbon nanotubes at temperatures ranging from 298 to 683 K. The structural characteristics of the coordination shells of ions are studied, including the ion-oxygen radial distribution functions, the coordination numbers, and the orientation distributions of the water molecules. Simulation results show that the first coordination shells of the five ions still exist in the nanoscale confinement. Nevertheless, the first coordination shell structures of cations change more significantly than those of anions because of the preferential orientation of the water molecules induced by the carbon nanotube. The first coordination shells of cations are considerably less ordered in the nanotube than in the bulk solution, whereas the change of the first coordination shell structures of the anions is minor. Furthermore, the confinement induces the anomalous behavior of the coordination shells of the ions with temperature. The first coordination shell of K+ are found to be more ordered as the temperature increases only in the carbon nanotube with the effective diameter of 1.0 nm, implying the enhancement of the ionic hydration with temperature. This is contrary to that in the bulk solution. The coordination shells of the other four ions do not have such behavior in the carbon nanotube with the effective diameter ranging from 0.73 to 1.00 nm. The easier distortion of the coordination shell of K+ and the match of the shell size and the nanotube size may play roles in this phenomenon. The exchange of water molecules in the first coordination shells of the ions with the solution and the ion diffusion along the axial direction of the nanotube are also investigated. The mobility of the ions and the stability of the coordination shells are greatly affected by the temperature in the nanotube as in the bulk solutions. These results help to understand the biological and chemical processes at the high temperature.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...