Effects of natural convection on thermal explosion in a closed vessel
Abstract
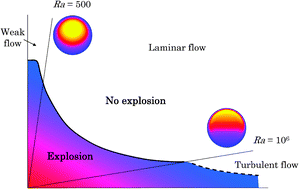
A new way of ascertaining whether or not a reacting mixture will explode uses just three timescales: that for chemical reaction to heat up the fluid containing the reactants and products, the timescale for heat conduction out of the reactor, and the timescale for natural convection in the fluid. This approach is developed for an nth order chemical reaction, A → B occurring exothermically in a spherical, batch reactor without significant consumption of A. The three timescales are expressed in terms of the physical and chemical parameters of the system. Numerical simulations are performed for laminar natural convection occurring; also, a theoretical relation is developed for turbulent flow. These theoretical and numerical results agree well with previous experimental measurements for the decomposition of

 Please wait while we load your content...
Please wait while we load your content...