Structure, spectrum and decomposition of the doubly charged ion C2N2++
Abstract
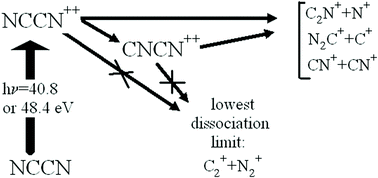
The electronic structure and fragmentation of the cyanogen dication formed by double photoionisation of the neutral NCCN molecule, are investigated by means of time of flight photoelectron–photoelectron coincidence (TOF-PEPECO) and photoelectron–photoion–photoion coincidence (PEPIPICO) techniques. Large scale ab initio computations at the cc-pVDZ/RCCSD(T) and cc-pVQZ/CASSCF levels of theory are performed in order to provide detailed structures, harmonic wavenumbers, vertical excitation energies and unimolecular decay pathways of the NCCN++ and CNCN++ isomers. Both theoretical and

 Please wait while we load your content...
Please wait while we load your content...