Formation and decay of tetrazane derivatives—a Car–Parrinello molecular dynamics study†
Abstract
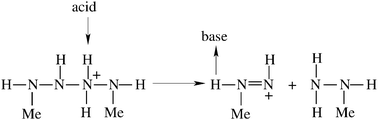
The complications during flight 510 of the Ariane Project were ascribed to problems in the upper stage engine that employs the bipropellant monomethylhydrazine (MMH) and

 Please wait while we load your content...
Please wait while we load your content...