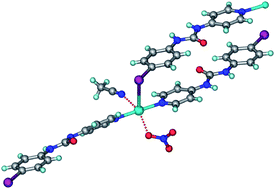
Silver–pyridyl complexes of N-4-X-phenyl-N′-4-pyridyl urea (X = Cl, Br, I, F, H; L1–L5) are crystalline solids of the formula [Ag(L)2](S)(NO3) (S = MeCN) for chloro, bromo and iodo ligands (1–3). Solid solution crystals 7–9 of bromo and iodo derivatives were prepared with L2 : L3 : mixed ligands ratio of 46 : 37 : 17, 65 : 19 : 16 and 94 : 0 : 6 (mixed = bromo–Ag–iodo) in the product. The significance of Ag⋯halogen interaction decreases in the expected order I > Br > Cl in isostructural crystal structures. Interestingly, the polar δ+ region of the C–X bond interacts with nitrate O− in a near-linear approach and the equatorial δ− region approaches Ag+ metal atom at a bent angle, consistent with charge polarization at the heavy halogens. The fluoro and protio ligand silver complexes 4–6 are quite different from structures 1–3. Urea–nitrate N–H⋯O hydrogen bonds have R22(8), R21(6), R43(14) and R44(16) cyclic motifs.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...