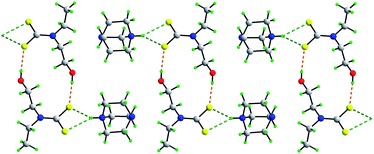
A range of supramolecular architectures is found in the title dithiocarbamate salts, each with hydrogen bonding functionality in the form of aτ least one hydroxyl group. A common feature in the crystal packing is the prevalence of charge-assisted O–H⋯S hydrogen bonding. In [NH4][S2CN(CH2CH2OH)2] (1), a 3-D network is found mediated by cooperative O–H⋯S, N–H⋯O and N–H⋯S hydrogen bonding. Reducing the hydrogen bonding functionality by replacing the ammonium cation in (1) by the 4-aza-1-azoniabicyclo(2.2.2)octanium cation to give [DABCO-H][S2CN(CH2CH2OH)2] (2), results in a 2-D array. Further reduction of the hydrogen bonding functionality, this time by substituting a CH2CH2OH with an alkyl group to give [DABCO-H][S2CN(CH2CH2OH)CH3] (3) and [DABCO-H][S2CN(CH2CH2OH)CH2CH3] (4) allows for the formation of 1-D supramolecular chains. The introduction of alkali metal cations rather than protic cations removes the possibility of the hydroxyl-O participating in hydrogen bonding interactions as these now coordinate the alkali metal. In the sodium trihydrate, Na[S2CN(CH2CH2OH)2]·3H2O (5), O–H⋯O hydrogen bonds are found along with charge-assisted O–H⋯S contacts so that a 3-D network results. Substituting a CH2CH2OH group with a n-propyl group gives Na[S2CN(CH2CH2OH)CH2CH2CH3]·2H2O (6) and yields a 2-D array. For the anhydrous K[S2CN(CH2CH2OH)2] (7) and Cs[S2CN(CH2CH2OH)2] (8) salts, the crystal packing is dominated by charge-assisted O–H⋯S hydrogen bonding giving 3-D network structures. The systematic analysis of the crystal packing patterns of these salts reveals the importance of charge-assisted O–H⋯S hydrogen bonding in stabilising these crystal structures.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...