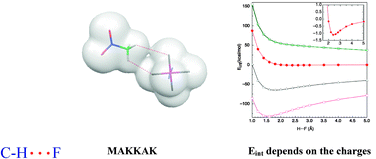
The nature of the C–H⋯F interactions in molecular crystals has been exhaustively reinvestigated, differentiating the case where both interacting fragments are neutral from the case where at least one fragment is charged. After selecting all complexes found in the Cambridge Structural Database which have short C–H⋯F interactions, an investigation was conducted on (a) their strength, (b) directionality, (c) properties of the H⋯F bond critical points, and (d) energetic components of the interaction energy. It is found that when the two fragments involved in the interaction are neutral, C–H⋯F interactions behave as weak hydrogen bonds: they have an interaction energy around −0.4 kcal mol−1, dominated by the electrostatic and dispersion components (the latter being stronger). When at least one fragment is charged, C–H⋯F interactions are also hydrogen bonds, only having interaction energies between −164 and 50 kcal mol−1, the electrostatic energetic component being their strongest one. The repulsive C–H⋯F interactions are produced between ions of the same sign and are not hydrogen bonds. The attractive interactions are placed well beyond the so-called covalent limit (−50 kcal mol−1) and thus have to be considered as members of the recently found ion–ion hydrogen bond class. The angular dependence of the C–H⋯F interactions in charged and neutral crystals is similar, with a maximum around 120° for ∠C–H⋯F angles. Calculations on isolated complexes show a collinear preference. Therefore, the lack of collinearity in crystals is due to the presence of other interactions in the solid. The similarity between the angular distribution of charged and neutral fragments is explained on the basis of the similar local density and molecular electrostatic potential that neutral and charged fragments present close to the C–H and F groups.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...