Silylmethyl-substituted cyclopropyl and other strained ring systems: cycloaddition with dipolarophiles
Abstract
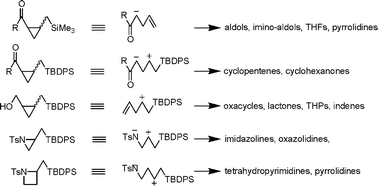
Small ring compounds represent a class of versatile building blocks in organic synthesis. Three- and four-membered ring carbo- and heterocycles are regarded as unique functional groups.

 Please wait while we load your content...
Please wait while we load your content...