Enantioselective organocatalyzed Henry reaction with fluoromethyl ketones†
Abstract
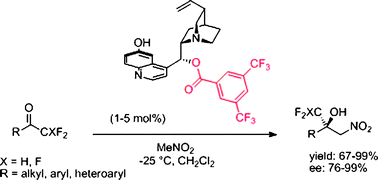
Remarkable generality in scope of new C9-benzoylcupreines bearing electron-withdrawing substituents for the nitroaldol condensation with fluoromethyl ketones is presented. Both tri- and difluoromethyl

 Please wait while we load your content...
Please wait while we load your content...