Highly enantioselective organocatalytic formation of a quaternary carbon center via chiral Brønsted acid catalyzed self-coupling of enamides†
Abstract
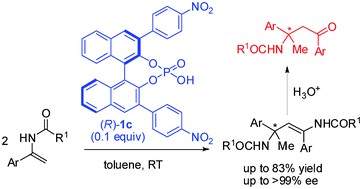
The enantioselective BINOL-phosphate catalyzed formation of a quaternary carbon center, bearing a N-atom has been achieved through the self-coupling reaction of enamides; the corresponding products have been isolated in up to >99% ee and their application for the synthesis of versatile synthetic building blocks—β-aminoketones—has been demonstrated.

 Please wait while we load your content...
Please wait while we load your content...