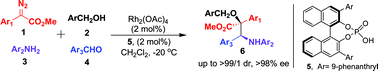
Selectivity control in enantioselective four-component reactions of aryl diazoacetates with alcohols, aldehydes and amines: an efficient approach to synthesizing chiral β-amino-α-hydroxyesters†
Abstract
Enantioselective four-component reactions of aryl diazoacetates with

 Please wait while we load your content...
Please wait while we load your content...