Generation of a triplet diradical from a donor–acceptor cross conjugate upon acid-induced electron transfer†
Abstract
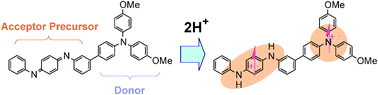
Enhancement of the electron acceptor ability of a para-quinodiimine unit by double protonation leads to the proton-induced intramolecular electron transfer from the donor unit to the cross-conjugated acceptor, giving rise to ground state triplet diradical reversibly.

 Please wait while we load your content...
Please wait while we load your content...