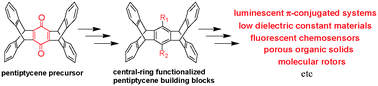
The progress of pentiptycene chemistry is reviewed. Pentiptycene belongs to the iptycene family and possesses a rigid, aromatic, and H-shaped scaffold. An important feature for pentiptycene vs. triptycene is the presence of a ‘sterically shielded’ central benzene ring. Such a feature has led to the use of pentiptycene as a conformational regulator and in the formation of functional molecules, including fluorescent chemosensors, molecular machines, low dielectric constant materials, and porous solids. The synthesis of these materials relies on central-ring prefunctionalized pentiptycene building blocks. A useful approach toward the preparation of these building blocks is the derivatization of pentiptycene quinone.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...