Pre-activation protocol leading to highly stereoselectivity-controllable glycosylations of oxazolidinone protected glucosamines†
Abstract
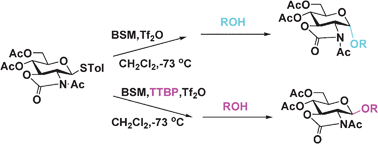
Under pre-activation glycosylation conditions, the 4,6-di-O-acetyl-N-acetyloxazolidinone protected donor afforded either excellent β- or α-stereoselectivity simply by means of the addition of hindered base TTBP or the absence of base, leading to the controllable stereochemistry of coupling reactions.

 Please wait while we load your content...
Please wait while we load your content...