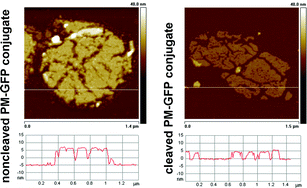
One of the key challenges in the construction of complex bionanotechnological building blocks and devices is the controlled linkage and release of biomolecular components to and from a biomolecular surface. Here we report a versatile, easy and universally applicable method for the reversible assembly of protein–protein conjugates. The process is demonstrated using green fluorescent protein (GFP) and purple membranes (PM) as model compounds. GFP was reversibly bound to PM patches which served as a biomolecular model surface. Due to its size in the micrometre range PM is, as far as its Brownian diffusion is considered, like a solid surface. PMs comprising the mutated bacteriorhodopsin BR-D36C were employed, where aspartic acid in position 36 was replaced with cysteine. The introduced cysteine is accessible from the cytoplasmic side of the membrane. The cysteine group was first functionalized with a nitrilotriacetic acid group (NTA) and then, after loading with Ni2+, histidine-tagged GFP was bound to the chemically modified PM surface via the well-known NTA-His6 complex. Binding and release of GFP from the PM surface was monitored by atomic force microscopy (AFM).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...