Experimental study of the excited-state properties and photostability of the mycosporine-like amino acid palythine in aqueous solution
Abstract
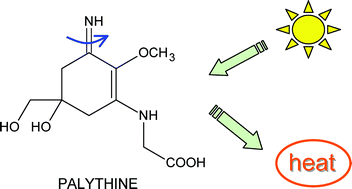
Characterization of the excited states of the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond may contribute to the rapid deactivation of this group of molecules.
N bond may contribute to the rapid deactivation of this group of molecules.

 Please wait while we load your content...
Please wait while we load your content...