Redox compounds influence on the NAD(P)H:FMN–oxidoreductase–luciferase bioluminescent system
Abstract
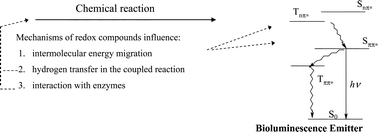
A review of the mechanisms of the exogenous redox compounds influence on the bacterial coupled enzyme system: NAD(P)H:FMN–oxidoreductase–luciferase has been done. A series of

 Please wait while we load your content...
Please wait while we load your content...