Synthesis, stereochemistry, and photochemical and thermal behaviour of bis-tert-butyl substituted overcrowded alkenes†
Abstract
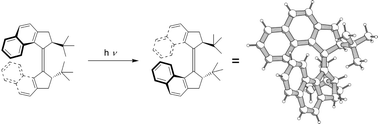
In order to study the structural limits in the design of molecular motors, a tert-butyl substituted analogue was prepared. The unexpected

 Please wait while we load your content...
Please wait while we load your content...