Synthetic chemistry-led creation of a difluorinated biaryl ether non-nucleoside reverse transcriptase inhibitor†
Abstract
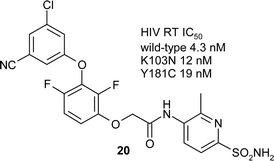
In the process of developing a series of novel, fluorinated biaryl ether NNRTIs, we fortuitously discovered derivative 20, which possesses excellent potency against both wild-type and clinically relevant mutations of the

 Please wait while we load your content...
Please wait while we load your content...