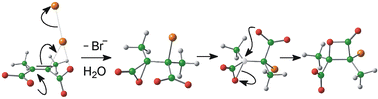
Structural analysis of the bromo-β-lactones obtained by addition of bromine to aqueous solutions of disodium 2,3-dimethylmaleate and 2,3-dimethylfumarate reveals stereochemistries opposite to those originally assigned in 1937: cisalkene yields erythrolactone, and transalkene yields threolactone. B3LYP/6-31+G(d) calculations using a PCM description of aqueous solvation confirm the validity of our proposed mechanism, in which the first-formed intermediate in each case is an α-lactone. The cyclic bromonium species is not an intermediate. An alternative pathway leading directly from cisalkene to cislactone, via an unusual frontside displacement mechanism, is over 20 kJ mol–1 higher in free energy. Hydrolysis of the bromo-β-lactones yields bromohydrins whose stereochemistries as determined by X-ray crystallography indicate stereospecific formation by acyl–oxygen cleavage of the lactone ring, again contrary to the original view.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...