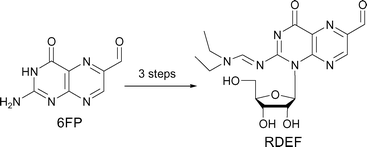
We demonstrated previously that 3-position-modified 6-formylpterin (6FP) derivatives produce reactive oxygen species (ROS) such as hydrogen peroxide (H2O2) from oxygen in the presence of NADH in the dark. It has been shown that 6FP derivatives markedly generate ROS, which gives rise to their particular physiological activities, such as induction of apoptosis in cellular and living systems, suggesting that such compounds provide a hint for the design of a ROS controlling agent in vivo. However, it is not well understood why such unique activities appear on chemical modification. In the present study, in order to see the effect on ROS generation activity in the dark by the modification of the 1-position in 6FP, we have developed a new synthetic procedure for nucleoside analogs of 6FP and prepared 1-(β-D-ribofuranosyl)-2-(N,N-diethylaminomethyleneamino)-6-formylpteridin-4-one (RDEF) and 1-(β-D-ribofuranosyl)-2-(piperidine-1-ylmethyleneamino)-6-formylpteridin-4-one (RPIF) in which the 1-position of 6FP is glycosylated. At pH 7.4, NADH was spontaneously oxidized to NAD+ in the presence of RDEF in the dark. Using electron paramagnetic resonance analysis coupled with the spin trapping technique, we show that O2 was converted to H2O2viasuperoxide anion radical (˙O2–) during this reaction. The modification of the 1-position of 6FP did not cancel ROS generation activities, which were demonstrated in 3-position-modified 6FPs. Since the 6FP derivatives developed in the present study have a ribose moiety, these compounds can be subjected to further derivatization, such as incorporation into oligonucleotides, oligosaccharides, proteins, or any other compounds that recognize and interact with specific biomolecules, and therefore would be useful in pharmaceutical investigations that need generation of appropriate and controllable amounts of ROS in vivo.

 Please wait while we load your content...
Please wait while we load your content...