Tuning of fluorescence properties of aminoterpyridine fluorophores by N-substitution†
Abstract
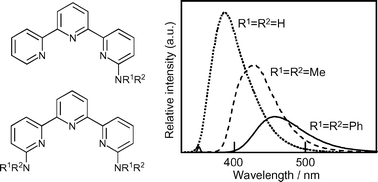
Several N-alkyl and N-phenyl derivatives of 6-amino- (1) and 6,6′-diamino-2,2′:6′,2″-terpyridine (6) were synthesized, and their fluorescence properties were studied. A successive red-shift was observed as the number of the N-substituted groups increased. It was also shown that the susceptivity of the fluorophores to a solvent varied considerably according to the mode of the N-substitution. While the monoamino-tpys 1–5 (tpy: 2,2′:6′,2″-terpyridine) suffered almost complete quenching of their fluorescence in

 Please wait while we load your content...
Please wait while we load your content...