Abstract
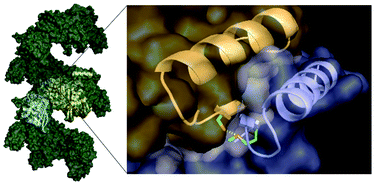
Bacterial RecA promotes the development and transmission of antibiotic resistance genes by self-assembling into an ATP-hydrolyzing filamentous homopolymer on single-stranded DNA. We report the design of a 29mer peptide based on the RecA N-terminal domain involved in intermonomer contact that inhibits RecA filament assembly with an IC50 of 3 µM.

 Please wait while we load your content...
Please wait while we load your content...