Carbanion reactivity, kinetic and equilibrium studies of σ-adduct formation and elimination in the reactions of 4-nitrobenzofurazan derivatives with nitroalkane anions†
Abstract
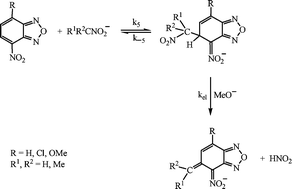
1 H NMR studies are reported of the reactions in [2H6]-DMSO of 4-nitrobenzofurazan, 2a, and its 7-chloro- and 7-methoxy-derivatives, 2b and 2c respectively, with anions derived from nitromethane, 3, nitroethane, 4, and 2-nitropropane, 5. The initial reactions result in σ-adduct formation by carbanion attack at the 5-position of 2a–c and in the case of reaction of 2a with 5 the adduct at the 7-position is also observed. These reactions may be followed by base catalysed elimination of nitrous acid to yield anionic alkene derivatives. Kinetic and equilibrium measurements of these reactions were made spectrophotometrically in methanol. The carbon nucleophilicities of the carbanions decrease in the order 3 > 4 > 5, as also found in their reactions with benzhydrylium cations, and are much lower than the nucleophilicities of some cyano-substituted carbanions. Comparison with corresponding σ-adduct forming reactions of 1,3,5-trinitrobenzene, TNB, show that here 2 and TNB have similar electrophilicity, although the value of the intrinsic rate coefficient ko = 0.05, for reaction of 2 is rather lower than that, ko = 0.20, for the TNB reactions. Literature data suggest that for reaction with a variety of nucleophiles 2 and TNB show similar electrophilicities. Measurements of the rates of elimination of nitrous acid from some 5-adducts in methanol catalysed by methoxide ions are reported. Values of rate constants may be influenced both by steric requirements at the reaction centre and by the electronic effects of the 7-substituent.

 Please wait while we load your content...
Please wait while we load your content...