Entering the leinamycin rearrangement via 2-(trimethylsilyl)ethyl sulfoxides
Abstract
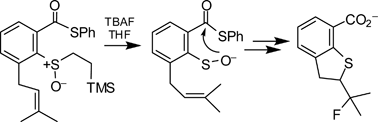
Attack of cellular thiols on the antitumor natural product leinamycin is believed to generate a sulfenate intermediate that undergoes subsequent rearrangement to a DNA-alkylating episulfonium ion. Here, 2-(trimethylsilyl)ethyl sulfoxides were employed in a fluoride-triggered generation of sulfenate anions related to the putative leinamycin-sulfenate. The resulting sulfenates enter smoothly into a leinamycin-type rearrangement reaction to afford an episulfonium ion alkylating agent. The results provide evidence that the sulfenate ion is, indeed, a competent intermediate in the leinamycin rearrangement. Further, the molecules examined here may provide a foundation for the design of functional leinamycin analogues that bypass the unstable and synthetically challenging 1,2-dithiolan-3-one 1-oxide moiety found in the natural product.

 Please wait while we load your content...
Please wait while we load your content...