Homopolymeric pyrrolidine-amide oligonucleotide mimics: Fmoc-synthesis and DNA/RNA binding properties†
Abstract
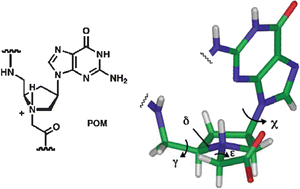
By chemically modifying or replacing the backbone of oligonucleotides it is possible to modulate the DNA and RNA recognition properties and fine-tune the physiochemical properties of oligomers. This is important because it challenges our understanding of natural nucleic acid structural and recognition properties and can lead to nucleic acid mimics with a wide range of applications in nucleic acid targeting, analysis or diagnostics. In this paper we describe the solid phase synthesis of pyrrolidine-amide oligonucleotide mimics (POMs) using Fmoc-peptide chemistry. This required the synthesis of adeninyl, cytosinyl, thyminyl and guaninyl pyrrolidine monomers, with Fmoc- and standard acyl-protecting groups on the exocyclic amino groups and nucleobases respectively. These monomers were used to synthesise several thyminyl and adeninyl POM pentamers, with modest coupling efficiency. The pentamers were purified by RP-HPLC, characterised by mass spectrometry and their DNA and RNA binding properties were investigated using UV thermal denaturation/renaturation experiments. This revealed that all the pentamers exhibit strong affinity for complementary nucleic acids. The further evaluation of longer mixed-sequence POMs is described in a second accompanying paper (R. J. Worthington et al., Org. Biomol. Chem., 2006, DOI: 10.1039/b613386j).

 Please wait while we load your content...
Please wait while we load your content...