Spiroketals via oxidative rearrangement of enol ethers†
Abstract
Oxidative rearrangement of cyclic enol ethers leads to α-alkoxyesters. In the presence of a neighboring spiroether, this approach provides a stereoselective access to spiroketals. A modified proposal for the biosynthesis of
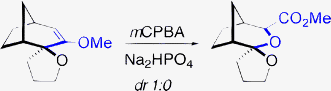

 Please wait while we load your content...
Please wait while we load your content...