The conformational preferences of thiohydroxamic acids (N-hydroxythioamides) are investigated by the density functional B3LYP/6-311++G(3df,3pd)//B3LYP/6-31G(d) method in this work. Unlike hydroxamic acids, the thione and thiol forms are found to be equally stable in the gas phase, and the reaction pathways for the interconversion between the thione and thiol forms have been deduced to involve rotation about the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond of the thiol tautomer in the rate-determining step. The effect of aqueous solvation on the reactions has also been investigated. It is found that inclusion of a few explicit water molecules in an implicit solvent calculation is necessary in order to accurately account for hydrogen bonding effects. Thiohydroxamic acids, like their hydroxamic acid analogues, are found to be N-acids, both in the gas phase and in aqueous solution.
N bond of the thiol tautomer in the rate-determining step. The effect of aqueous solvation on the reactions has also been investigated. It is found that inclusion of a few explicit water molecules in an implicit solvent calculation is necessary in order to accurately account for hydrogen bonding effects. Thiohydroxamic acids, like their hydroxamic acid analogues, are found to be N-acids, both in the gas phase and in aqueous solution.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond of the
N bond of the 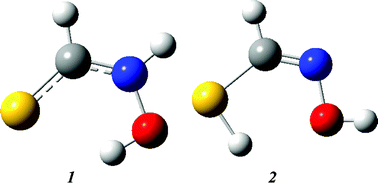

 Please wait while we load your content...
Please wait while we load your content...