A monoaqua zinc complex. Unique acid dissociation behaviour
Abstract
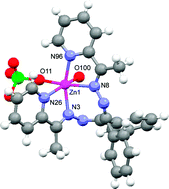
Using the 1 : 2 condensate of benzil dihydrazone and 2-acetylpyridine as the ligand L, two complexes of zinc, [ZnL(CH3COO)]PF6 (1) and [ZnL(H2O)ClO4]ClO4·H2O (2), are synthesised from Zn(CH3COO)2·2H2O and Zn(ClO4)2·6H2O, respectively. From X-ray crystallography, both the complexes are found to be single helical with the metal in distorted octahedral N4O2 environment. In 1, the two oxygen atoms come from the bidentate acetate while 2 is a monoaqua complex with a perchlorate anion bound to the metal in monodentate fashion. The perchlorate in 2 is not at all weakly bound [Zn–O(perchlorate) 2.256(4) Å]. Still in acetonitrile solution, the coordinated perchlorate ion dissociates upon deprotonation [reaction (i)].

 Please wait while we load your content...
Please wait while we load your content...