Anodic behaviour of methylidene-cyclopentadiaryl derivatives: cyclic voltammetry and theoretical study†‡
Abstract
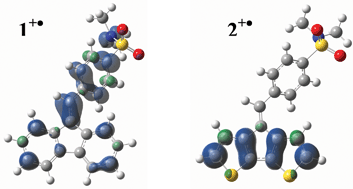
Electrochemical behaviour of a functionalized fluorenylidene 1 is studied in comparison with a cyclopentadithiophene analogue 2. The influence of the main aromatic group on the electropolymerization ability is reported. We demonstrated that the lack of sulfur atoms in the monomer structure leads to an absence of

 Please wait while we load your content...
Please wait while we load your content...