Aerobic oxidation of alcohols and alkylaromatics with dioxygen catalysed by N-hydroxyphthalimide with vanadium co-catalysts
Abstract
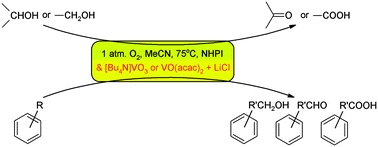
[Bu4N]VO3 or a combination of vanadium complexes with chloride salts are attractive co-catalysts for oxidation reactions of organic compounds with dioxygen catalysed by N-hydroxyphthalimide. The role of the chloride additive relates to the formation of more labile six-coordinate vanadium complexes. The same effect is also observed with amines. Two catalytic systems based on NHPI/VO(acac)2 with LiCl or [Bu4N]Cl and NHPI/[Bu4N]VO3 are most effective in oxidation reactions of primary and secondary alcohols, but oxidation of alkylaromatics proceeds with conversion rates of from 14.4% in the case of toluene to 67.0% in the case of ethylbenzene and with different selectivities, dependent on the structure of the substrate.

 Please wait while we load your content...
Please wait while we load your content...