Syntheses and photophysics of new phthalocyanine derivatives of zinc, cadmium and mercury
Abstract
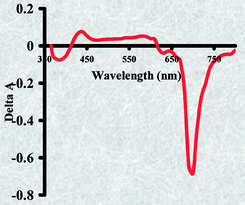
The syntheses of novel tetra{2,(3)-pyridyloxyphthalocyaninato} complexes of zinc, cadmium and mercury (TPyZnPc, TPyCdPc and TPyHgPc) are presented. Their spectral and photophysical properties (as well as those of their unsubstituted counterparts: ZnPc, CdPc and HgPc) are investigated. TPyZnPc and TPyCdPc are aggregated in non-coordinating solvents such as chloroform, while TPyHgPc is demetallated, as evident from their respective absorption spectra. The trends in fluorescence (ΦF), triplet (ΦT) and singlet oxygen (ΦΔ) quantum yields are explained in terms of relative strengths of spin–orbit coupling induced by the respective central metal ions in the complexes. The effect of the pyridyloxy substituents is a decrease in ΦF and an increase in ΦT values. The complexes are less fluorescent in DMSO but possess higher ΦT, triplet lifetimes (τT) and ΦΔ therein.

 Please wait while we load your content...
Please wait while we load your content...