An integrated system for enzymatic cleavage and electrostretching of freely-suspended single DNA molecules†‡
Abstract
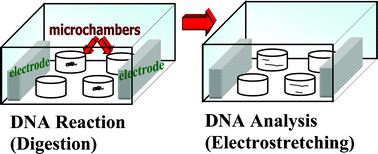
A novel polyacrylamide gel-based femtolitre microchamber system for performing single-molecule restriction enzyme assay on freely-suspended DNA molecules and subsequent DNA electrostretching by applying an alternating electric field has been developed. We attempted the integration by firstly initiating restriction enzyme reaction on a fluorescent-stained λDNA molecule, encapsulated in a microchamber, using magnesium as an external trigger. Upon complete digestion, the cleaved DNA fragments were electrostretched to analyze the DNA lengths optically. The critical parameters for electrostretching of encapsulated DNA were investigated and optimum stretching was achieved by using 1.5 kHz pulses with electric field strength in the order of 103 V cm−1 in 7% linear polyacrylamide (LPA) solution. LPA was adopted to minimize the adverse effects of ionic thermal agitation on molecular dielectrophoretic elongation in the microchamber. In our experiments, as the fragments were not immobilized throughout the entire protocol, it was found from repeated tests that digestion always occurred, producing the expected number of cleaved fragments. This versatile microchamber approach realized direct observation of these biological reactions on real-time basis at a single-molecule level. Furthermore, with the employment of porous polyacrylamide gel, the effective manipulation of DNA assays and the ability to combine conventionally independent bioanalytical processes have been demonstrated.

 Please wait while we load your content...
Please wait while we load your content...