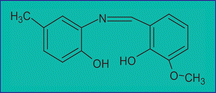
A simple, easy to use and selective spectrofluorimetric method for the determination of trace levels of aluminium has been developed. A new Schiff base, N-o-vanillidine-2-amino-p-cresol (OVAC), has been synthesized and its fluorescence activity with aluminium investigated. Based on this chelation reaction, a spectrofluorimetric method has been developed for the determination of aluminium in samples buffered at pH 4.0 using acetic acid–sodium acetate. The chelation reaction between Al(III) and N-o-vanillidine-2-amino-p-cresol was very fast, requiring only 20 min at room temperature to complex completely. The excitation and emission wavelengths were 423.0 and 553.0 nm, respectively, at which the OVAC–Al complex gave the maximum fluorescence intensity at pH 4.0 in a 50% ethanol–50% water medium. The interference from fluoride ions was minimized by the addition of Be2+. Other ions were found not to interfere at the concentrations likely to be found in natural waters. Under these conditions, the calibration plot was linear up to 1000 μg L−1 (r = 0.999). The limit of detection (3σ) for the determination of Al(III) was 0.19 μg L−1 and the precision for multiple determinations of 3 ng mL−1 Al(III) prepared in ultra-pure water was found to be 0.29% (n = 16). The Schiff base ligand could be used to determine ultra-trace aluminium from natural waters. Analysis of environmental certified reference materials showed good agreement with the certified values. The procedure was found to be equally applicable to both fresh water and saline solutions (including sea water) using either normal external calibration or the standard additions method.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...