The replacement of an acetate function of the macrocyclic DOTA4−
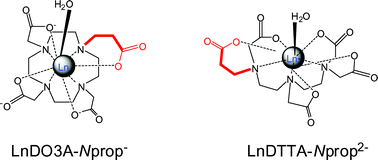
(DO3A-Nprop4−) or the acyclic DTPA5− in terminal position (DTTA-Nprop5−) has been recently shown to result in a significant increase of the water exchange rate on the Gd3+ complexes, which makes these chelates potential contrast agents for MRI applications. Here, two novel and straightforward synthetic routes to H4DO3A-Nprop are described. Protonation constants of DO3A-Nprop4− and stability constants with several alkaline earth and transition metal ions have been determined by potentiometry. For each metal, the thermodynamic stability constant is decreased in comparison to the DOTA chelates. The formation reaction of LnDO3A-Nprop− complexes (Ln = Ce, Gd and Yb) proceeds via the rapid formation of a diprotonated intermediate and its subsequent deprotonation and rearrangement in a slow, OH− catalyzed process. The stability of the LnH2DO3A-Nprop* intermediates is similar to those reported for the corresponding DOTA analogues. The rate constants of the OH− catalyzed deprotonation step increase with decreasing lanthanide ion size, and are slightly higher than for DOTA complexes. The kinetic inertness of GdDTTA-Nprop2− was characterized by the rates of its exchange reactions with Zn2+ and Eu3+. The rate of the reaction between GdDTTA-Nprop2− and Zn2+ increases with Zn2+ concentration, while it is independent of pH, implying that the exchange takes place predominantly via direct attack of the metal ion on the complex. In the Eu3+ exchange, the rate decreases with increasing concentration of the exchanging ion which is accounted for by the transitional formation of a dinuclear GdDTTA-NpropEu+ species. The kinetic inertness of the monopropionate GdDTTA-Nprop2− is decreased in comparison to GdDTPA2−: all rate constants, characterizing the dissociation reaction via either proton- or metal-catalyzed pathways being higher by 1–2 orders of magnitude. Similarly, a study of the acid-catalyzed dissociation of the macrocyclic CeDO3A-Nprop− showed a partial loss of the kinetic inertness with regard to the tetraacetate derivative CeDOTA−.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...