Formation of a triply bridged µ-oxo diiron(iii) core stabilized by two deprotonated carboxamide groups upon photorelease of NO from a {Fe–NO}6 iron nitrosyl†
Abstract
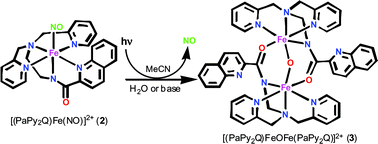
The iron nitrosyl [(PaPy2Q)Fe(NO)](ClO4)2 (2), derived from the quinoline-based ligand PaPy2QH (N,N-bis(2-pyridylmethyl)amine-N-ethyl-2-quinoline-2-carboxamide, where H is dissociable proton) has been characterized by spectroscopy and X-ray diffraction techniques. The 1H NMR spectrum (S = 0 ground state) and νNO value of 1885 cm−1 indicate that 2 is a {Fe–NO}6 nitrosyl. Although 2 is stable in the dark, exposure of an acetonitrile solution of 2 (λmax = 510 nm) to light in the visible range causes rapid release of NO and formation of the solvato species [(PaPy2Q)Fe(MeCN)](ClO4)2 (6). Quantum yield (Φ) measurements indicate that 2 is a more efficient NO donor (Φ = 0.258) than [(PaPy3)Fe(NO)](ClO4)2 (1, Φ = 0.185), a complex derived from a similar but pyridine-based ligand. Interestingly, when the photoproduct 6 is exposed to water or a small amount of base, the triply bridged diiron(III) species [(PaPy2Q)FeOFe(PaPy2Q)](ClO4)2 (3) forms in good yield. This species can be independently synthesized from aerobic oxidation of the Fe(II) species [(PaPy2Q)Fe(MeCN)](ClO4) in acetonitrile. The structure of 3 reveals a unique Fe(III)–O–Fe(III) link supported by two (η2,µ2)µ-![[upper bond 1 start]](https://www.rsc.org/images/entities/char_e010.gif) NCO
NCO![[upper bond 1 end]](https://www.rsc.org/images/entities/char_e011.gif) bridges derived from the carboxamido groups of the two (PaPy2Q)Fe(III) moieties.
bridges derived from the carboxamido groups of the two (PaPy2Q)Fe(III) moieties.

 Please wait while we load your content...
Please wait while we load your content...