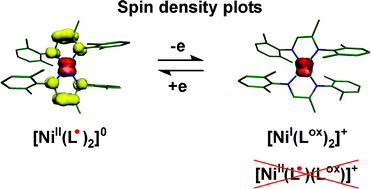
The reaction of 2 equivalents of 2-methyl-1,4-bis(2,6-dimethylphenyl)-1,4-diaza-1,3-butadiene, (1LOx)0, or 2-methyl-1,4-bis(2,6-diisopropylphenyl)-1,4-diaza-1,3-butadiene, (2LOx)0, in diethyl ether or n-hexane with 1 equivalent of Ni(cdt) where (cdt)0 is the ligand cyclododecatriene affords dark red, diamagnetic precipitates of [NiII(1L˙)2] (1) and of [NiII(2L˙)2] (3). The ligands (1L˙)1− and (2L˙)1− are the one-electron reduced, monoanionic π radicals of the above neutral 1,4-diaza-1,3-butadienes. 1 and 3 have been structurally characterized by X-ray crystallography; both possess a distorted tetrahedral geometry where the dihedral angle θ between the two metallacycles Ni–N–C–C–N is 47.9° and 53°, respectively, (θ = 0° for square planar and 90° for a regular tetrahedral geometry). The reaction of 1 and 3 with 1 equivalent of ferrocenium hexafluorophosphate gives the paramagnetic (St = 1/2) complexes 2 and 4, respectively: [NiI(1LOx)2](PF6) (2), [NiI(2LOx)2](PF6) (4). Their EPR spectra indicate the presence of a central Ni(I) ion (d9; SNi = 1/2). Thus, the one-electron oxidation of 1 and 3 by [Fc]PF6 induces an intramolecular one-electron reduction of the central Ni ion and a concomitant one-electron oxidation of the second π radical (L˙)1−
→ (LOx)0 + e. Broken symmetry DFT calculations (B3LYP) corroborate the correctness of the electronic structure descriptions of 1–4. The reaction of (1LOx)0 with NiI2 (1 : 1) in tetrahydrofuran yields tetrahedral [NiII(1LOx)I2] (5) with an St = 1 ground state.

 Please wait while we load your content...
Please wait while we load your content...