Increasing the solubility of strong reducing agents containing Mo24+ units and alkyl-substituted guanidinate ligands†‡
Abstract
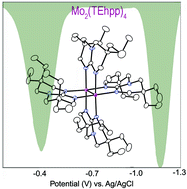
Six very soluble paddlewheel compounds containing Mo2n+ units, n = 4, 5, 6, and two alkyl-substituted bicyclic guanidinate ligands have been synthesized. The quadruply bonded complexes with n = 4, Mo2(TMhpp)4 and Mo2(TEhpp)4, (TMhpp = the anion of 3,3,9,9-tetramethyl-1,5,7-triazabicyclo[4.4.0]dec-4-ene and TEhpp = the anion of 3,3,9,9-tetraethyl-1,5,7-triazabicyclo[4.4.0]dec-4-ene) are easily oxidized. The electrode potentials in THF are −1.08 and −1.17 V vs. Ag/AgCl, respectively, for the Mo25+/4+ couple. These potentials are in accord with the low ionization potentials for the quadruply bonded compounds. Because of the high solubility of the Mo24+ compounds in most common organic solvents they are attractive candidates for use as strong reducing agents in homogeneous systems.

 Please wait while we load your content...
Please wait while we load your content...