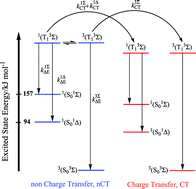
Photophysical properties in dilute acetonitrile solution are reported for a number of iridium(III) and rhenium(I) complexes. The nature of the lowest excited state of the complexes under investigation is either metal-to-ligand charge transfer (3MLCT) or a ligand centred (3LC) state. Rate constants, kq, for quenching of the lowest excited states by molecular oxygen are in the range 1.5 × 108 to 1.4 × 1010 M−1 s−1. Efficiency of singlet oxygen production, fΔT, following oxygen quenching of the lowest excited states of these complexes, are in the range of 0.27–1.00. The rate constants and the efficiency of singlet oxygen formation are quantitatively reproduced by a model that assumes the competition between a non-charge transfer (nCT) and a CT deactivation channel. The balance between CT and nCT deactivation channels, which is described by the relative contribution pCT of CT induced deactivation, is discussed. The kinetic model is found to be successfully applied in the case of quenching of the excited triplet states of coordination compounds by oxygen in acetonitrile, as was proposed for the quenching of π–π* triplet states by oxygen.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...